

Levi Olmstead


Quality management systems (QMS) have emerged as essential quality assurance tools that enable product excellence and compliance across different industry sectors.
As technology changes how companies in industries like food and beverage, pharmaceutical, medical device, and aviation operate, compliance and quality control become digitalized and automated. Companies that take products to market that are high-risk require QMS technology to help them compete, keep them compliant, allow them to ship safe products, and enable them to achieve their business objectives
In this article, we define quality management systems and their core features. Whether your organization is in manufacturing, healthcare, or any sector requiring stringent quality control, understanding the nuances of these systems is key to optimizing your Q/A, compliance, and overall operations. We’ll also explore the best QMS software based on our research, their market presence, each platform’s core offerings, their review rating, and more.
A quality management system (QMS) is a software platform that empowers organizations to stay on top of quality operations and policies for developing products and services. Quality management systems are essential to product development lifecycles in regulated industries such as food and beverages, healthcare, and manufacturing.
QMS software is used to streamline tasks such as:
For example, you can use QMS software to manage the creation, approval, and tracking of all documents required for industry standards and compliance requirements. Without QMS software, organizations are more susceptible to being slowed down by paper-based documents, manual workflows, and siloed information.
Not all QMS software has the same features and capabilities. Different QMS platforms are designed for industry-specific personas that address unique challenges to specific sector compliance guidelines and regulations.
A few of the more common QMS systems designed for industry-specific sectors and verticals include:
While different, industry-specific QMS software each has different features depending on what type of customers they service, there are still core QMS features found across all QMS platforms. Here is a list of core QMS features:
Software clicks better with Whatfix's digital adoption platform
Enable your employees with in-app guidance, self-help support, process changes alerts, pop-ups for department announcements, and field validations to improve data accuracy.
To help you on your QMS buying journey, we’ve highlighted the best QMS vendors based on their core features and overall perception in the QMS space. We’ve included an overview of each QMS product, review ratings from third-party websites, and the core industries each product serves.
We also placed these QMS vendors into groups based on their core industry, sector, and vertical niche, including:

SAP ERP’s QM module provides a quality management solution that enables enterprises across industries to streamline quality control processes with its robust set of enterprise resource management modules, like human resources management, procurement processes, finance tracking, supply chain management, and more. These cross-functional integrations give large organizations a single platform to manage day-to-day operations and quality assurance tasks across different departments, including non-quality and control processes.
The SAP platform is complex and highly customizable, making it a good choice for enterprises that want granular control over their operational workflows and strong security measures. You can use its quality management tools to create and execute quality inspection plans, manage quality control processes and values, maintain data for test equipment, and produce quality certificates that confirm products that meet quality requirements.

Alis QI is a QMS for manufacturers that want to strengthen their technology stack with automation and system integrations. Its no-code platform allows organizations to manage implementation and ongoing maintenance with minimal IT dependencies. AlisQI supports integrations with ERP tools like SAP, JD Edwards, and Dynamics. Beyond native integrations, teams can configure their APIs and webhooks for additional connections.
Its document management system allows manufacturers to automate Q/A workflows like review processes, edits, submissions, and permission controls. With AlisQI, teams can simplify quality manuals using flow charts to visualize processes quickly and attribute the proper documentation at the proper stages. You can start this process by uploading all existing quality manuals into the platform and begin using features like assigning owners, setting revision terms, and automating notifications.
Another feature of AlisQI is its ability to make quality control data actionable, so teams don’t have to use Excel for complex calculations. The platform is a Statistical Process Control (SPC) software with built-in tools to collect and analyze data. You can import data directly from Excel and create forms and tables for different quality control use cases.

ETQ’s quality management platform supports various industries, including aerospace, energy, and furniture. This cloud-based solution is modular and customizable. Organizations can browse and choose from over 40 applications to best fit their unique quality control processes. ETQ’s out-of-the-box applications include document control, training management, CAPA, audit management, change management, and materials management.
Businesses can also configure the platform with advanced applications tailored to specific industries. For example, apps have features geared toward supply chain quality, life sciences compliance, and lab investigations.
Its native applications allow teams to manage relationships with suppliers and business partners. You can use its Production Part Approval Process (PPAP) to streamline change approval workflows and sync this data into third-party systems like SAP and Oracle. Teams can also automate supplier management with real-time tracking of goods, supplier compliance, and supplier rating information.

Veeva Vault QMS enables life science and pharmaceutical companies with its quality management application that enables its customers to manage their entire quality assurance, control, and compliance processes.
This includes providing tools to control and manage business processes, documentation, SOPs, supplier management, creating audit trails, tracking and reporting metrics like quality events and deviations, staying compliant with industry regulations, and manufacturing high-quality products. It empowers organizations to improve efficiency, visibility, and control in quality management with its unified cloud solution that centralizes all processes, documents, and data.

MasterControl’s platform is used by organizations in regulated industries, such as healthcare, pharmaceuticals, and biologics. You can use it to securely store and manage digital documents, ensure regulatory compliance for change control processes, conduct employee training programs, and prepare for internal or external audits. The platform suits companies of all sizes looking for a single platform to digitize and automate compliance management for heavily regulated product development.
Companies typically use MasterControl’s digital document management solution to digitalize manual paper-based processes. It’s a connected platform that allows teams to streamline documentation processes with corresponding version control approvals, archiving, audit trails, and training tasks that automatically pop up to reflect document changes.
With its quality event management solution, teams can use MasterControl’s no-code workflow builder and prebuilt to design custom processes and boost efficiency with automated triggers. You can enhance workflows with in-app or email notifications, task lists, and the ability to automatically create Corrective and Preventive Actions (CAPA) from specific events — like customer complaints, audits, and deviations.

Qualio’s cloud-based QMS platform helps businesses in the life sciences industry centralize digital documentation and auditing processes. This solution is tailored toward companies that produce medical devices, pharmaceuticals, biotech, and more.
Its platform has features designed for organizations to stay on top of International Organization for Standardization (ISO) requirements, FDA clearance goals, and compliance standards when bringing medical devices to markers with different regulations, like the European Union and the United Kingdom. Qualio’s core features include document management, change control, audits, CAPAs, and supplier management. Companies in the life sciences industry can also use the platform to automate design control workflows when developing compliant medical devices.
Teams can use Qualio to eliminate communication siloes between departments by centralizing documentation from tools like Jira, Azure DevOps, and TestRail. You can also instantly create product releases and view a summary of product documentation generated at different stages of the product lifecycle, allowing you to be audit-ready at all times without any paper documentation or having to backtrack your work manually.

ZenQMS is a platform created by life sciences professionals with contextual knowledge of the manufacturing process and challenges specific to the life science vertical. It’s designed to be easy to manage compliance with “good practice” (GxP) quality guidelines and ISO requirements. It’s suitable for small and large life sciences organizations, with customers ranging from pre-clinical to commercial products. Given this target market, ZenQMS meets all relevant regulatory requirements for these industries.
It has capabilities across different quality management modules for document management, change control, employee training, audits, and issue management. All modules are accessible at a fixed price for turnkey implementation.
ZenQMs is tailored to help organizations get started with ISO 9001 quality management. Because an external body carries out this certification, it becomes a complex project with many moving parts. ZenQMS can manage documents with compliant electronic signatures, track staff training, assign CAPAs, and manage external services. The platform’s modules are customizable so teams can design processes with unique forms, stages, workflows, and dashboards.

Arena is a QMS platform suitable for teams that develop products with a distributed workforce and depend on global supply chains. It helps organizations stay on top of regulatory compliance for complex electronics products and medical devices across different regions, with features to ensure adherence to standards such as FDA, ISO, International Traffic in Arms Regulation (ITAR), and Export Administration Regulations (EAR).
Arena QMS also has additional capabilities tailored toward streamlining complex medical device manufacturing processes, including standard operating procedures (SOP) management, device history files, and device master records.
It enables organizations to centralize documentation and workflows for quality assurance, design controls, training programs, engineering, and audits. Arena also has a software validation solution that teams can seamlessly integrate with their QMS processes.
With cloud-based software validation, organizations can work with comprehensive ready-to-use documentation and reports, risk assessment and impact analysis notifications, and ongoing support from validation experts. On top of its validation solution, manufacturers can use Arena’s 3D visualization features to support collaboration and approvals involving computer-aided design (CAD) files.

Greenlight Guru is a cloud-based QMS solution tailored to the medical device industry, helping teams achieve compliance with global standards such as FDA and ISO. It’s designed to help medical device companies bring products to market fast and efficiently with less risk.
Greenlight Guru’s QMS software includes key features such as document management, training management, audit and CAPA management. Its signature design controls, risk management, and AI-powered risk intelligence help fast-track medical device development while ensuring compliance. It also offers end-to-end traceability throughout the product lifecycle to help automate processes, break down information silos, and reduce risk of noncompliance.
The software’s pre-validated workflows align with FDA and ISO standards, helping teams focus on innovation and maintaining quality without the burden of extensive software validation. Paired with a library of audit-tested QMS templates and backed by decades of in-house industry expertise, Greenlight Guru not only aids companies in modernizing their quality system but also improves operational efficiencies by scaling quality throughout their organization.

Windchill’s QMS platform enables manufacturers to maintain quality and compliance across the product lifecycle management process. Its platform is built for companies with a distributed workforce.
It’s cloud-based and adopts an open architecture that is easy to customize and integrate with other enterprise solutions using APIs and native integrations. You can use Windchill’s integrations to access digital product traceability, electronic design automation integrations, standards-based data exchange, and more.
Windchill QMS introduces more flexibility with add-on applications for different industries, roles, and functions. These add-ons include tools for mechanical designers, quality and compliance managers, reliability engineers, and more.
Windchill has additional offerings pre-configured with quality management features for companies developing products in the medical devices, aerospace and defense, and retail industries. For example, their Medical Device Suite (MDS) offerings are designed with ISO 13485 processes, while their Aerospace and Defense Module (WAD) allows companies to use Commercial and Government Entity codes.

TrackWise is a cloud-based QMS platform that uses a modular approach to help manufacturers integrate their quality processes into a single solution. TrackWise is built on Salesforce, making it easy to configure with Salesforce’s ecosystem and third-party integrations, including form builders, the SFDC Lightning interface, and countless more.
Its core QMS features include document management, supplier quality management, complaint management, risk management, and employee training. With these features, teams can automate non-conformances, complaint assessments, compliance tracking, and collaboration with suppliers for onboarding, audits, and incidents.
TrackWise uses AI to empower organizations with decision-making workflows powered by machine learning and NLP. Called Qualitywise.ai, this AI-powered feature provides automatic recommendations and pattern recognition to enable better quality and control decision-making. Organizations can use this AI to suggest quality categories and risks based on the complaints they receive to reduce the time spent investigating these incidents and events.

Ideagen is a quality management platform for organizations in heavily regulated industries to manage cross-functional collaboration and expedite operations for governance, risk, and compliance processes. It’s a cloud-based solution that enterprises can easily customize and scale to manage high volumes of processes and strict data protection policies.
The platform is well-versed in compliance and quality management challenges for each industry, with a suite of capabilities ranging from document management to incident tracking, risk analysis, and business intelligence.
Ideagen QMS is a robust platform focusing on four key areas — real-time reporting, traceable quality control systems, streamlined processes, and mobile incident reporting. With these solutions, you can use the platform’s modular infrastructure to build a centralized experience for all quality management data.
Beyond the platform’s features for documentation and collaboration, organizations can also integrate Ideagen’s QMS with its solutions for environment, health and safety (EHS) performance and operations. This includes capabilities like real-time EHS data and KPI tracking, out-of-the-box reports and dashboards, behavior-based safety modules, and a mobile app for on-the-go access to safety and risk management features.

Intellect is a QMS platform that uses AI technology for advanced workflow management. The tool integrates with OpenAI so companies can use text-based generative AI to verify procedures with compliance standards, look up answers for ISO and FDA audits, and draft documents or training materials.
Its platform uses AI to synthesize data into relevant metrics, pattern recognition, and performance benchmarking. These AI features are suitable for applications in document management, skill gap analysis, employee training, vendor management, risk management, and more.
Intellect’s core QMS features aim to help organizations in heavily regulated industries manage audits, monitor risks, collaborate across functions, and operationalize compliance workflows. It has standard capabilities such as document control, change management, CAPA, and a reporting dashboard.
Organizations can further add to these out-of-the-box functionalities with add-on applications. Popular apps include contract management, compliance management, conformance, supplier management, and product quality.

QT9 is a quality management platform optimized for ISO and FDA compliance processes. It has over 20 modules that organizations can use to configure the QMS experience across geographies, departments, and data sources. You can use these modules to implement solutions for the customer experience, document control, ISO management, supplier relationships, product development, maintenance, and management.
One of the platform’s most popular features is its Customer Web Portal. With this module, manufacturers can automate customer communication to alert them about forms and approvals for quality records. Collect customer feedback with surveys that can be attached directly to the platform. This portal also minimizes fragmented communication threads, such as manual emails that can be hard to record in one place. Instead, customers can upload documents into the portal and communicate directly with your team within the portal. The platform also has a similar portal for suppliers and distributors.
Whatfix helps organizations maximize their QMS software investment and elevate quality management strategies with contextual in-app onboarding, reinforcement training, real-time end-user support, and user behavioral analytics and event tracking.
Whatfix enables organizations with the technology to:
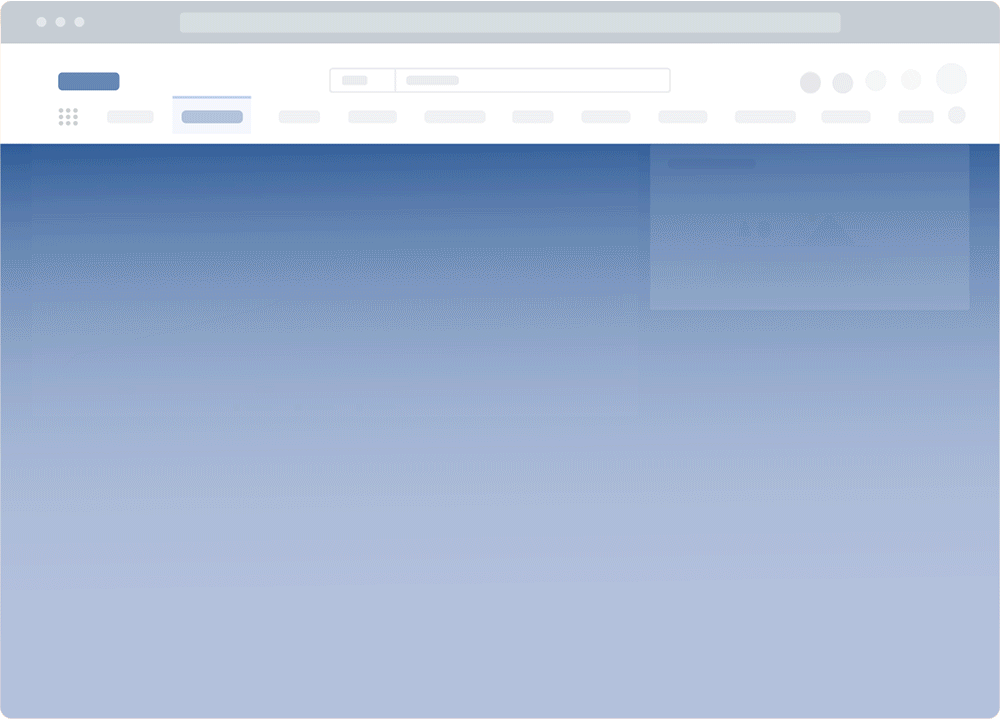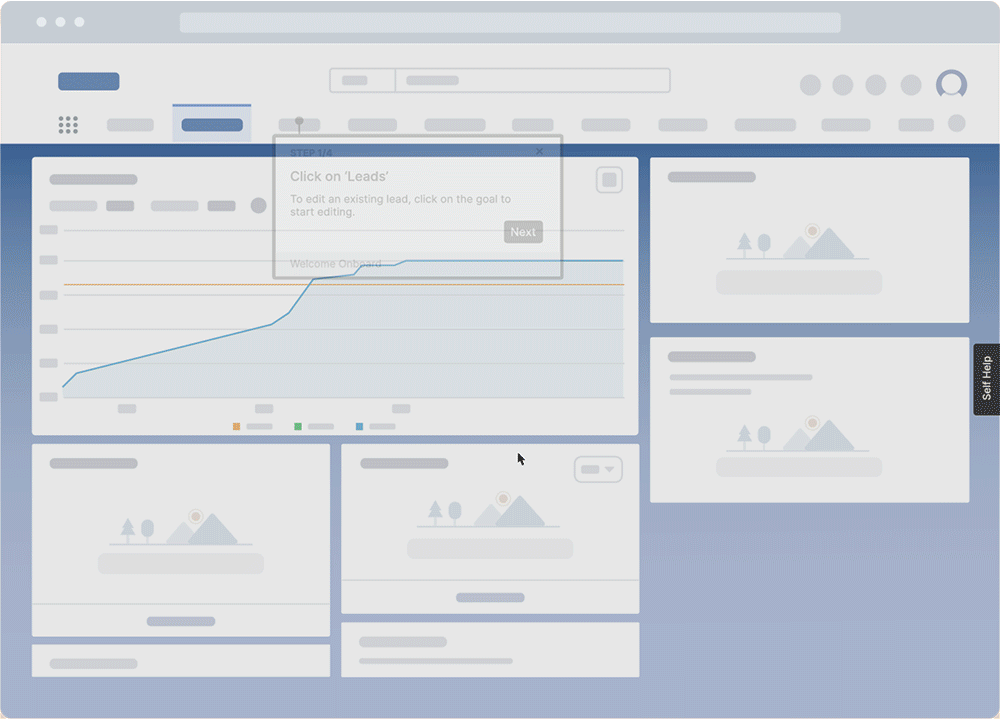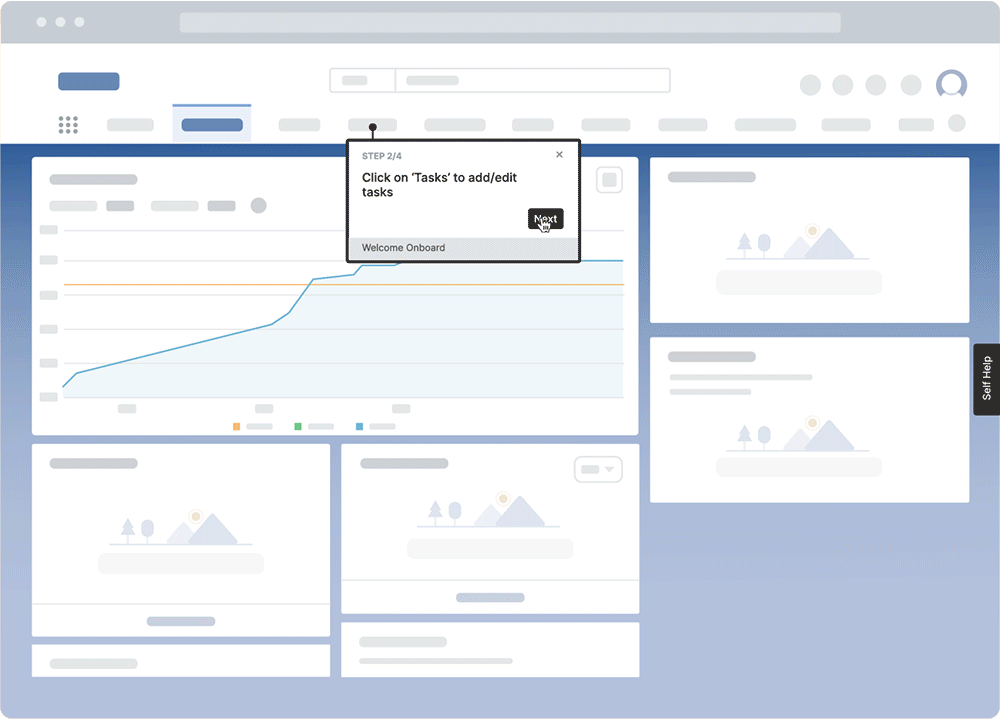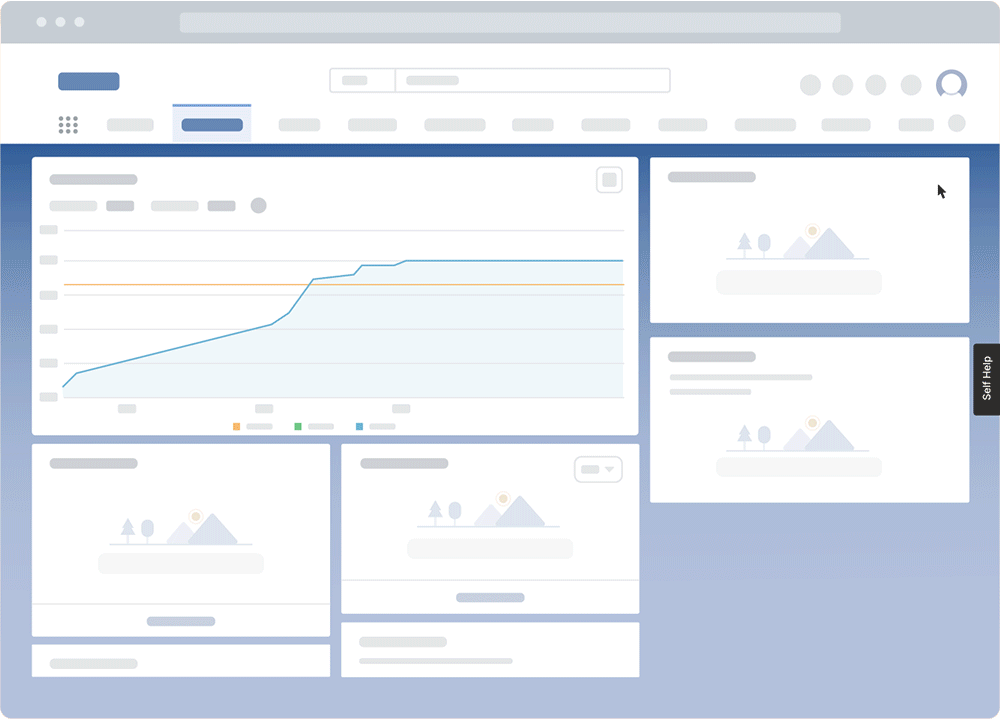
With a digital adoption platform like Whatfix, enable your employees with in-app guidance and contextual self-help IT support to accelerate the adoption of new software implementations, employee onboarding, change initiates, and more. Whatfix’s no-code editor enables IT teams with a no-code editor to create product tours, interactive walkthroughs, task lists, smart tips, pop-ups, self-help wikis, and more. Analyze and measure user engagement and software usage to identify friction points, measure digital adoption, and improve employee digital experiences.
Thank you for subscribing!